MORGANTOWN, W.Va. – The WVU Rockefeller Neuroscience Institute was first institution in the United States to enroll a patient in the Route 92 Medical, Inc. SUMMIT MAX clinical trial. SUMMIT MAX is a randomized, controlled, multicenter trial to evaluate the performance of its next generation Monopoint™ Reperfusion System versus currently available aspiration catheter technology.
The trial’s first patient was enrolled at Auckland City Hospital in New Zealand, followed shortly by a patient at the WVU Rockefeller Neuroscience Institute.
“We are happy to become the first U.S. site to enroll a patient in the SUMMIT MAX clinical trial evaluating the Monopoint System,” Ansaar Rai, M.D., WVU Rockefeller Neuroscience Institute professor and Neuroradiology Department chair, said. “Randomized clinical trials such as these offer high levels of evidence and are critical in advancing the field of endovascular stroke therapy.”
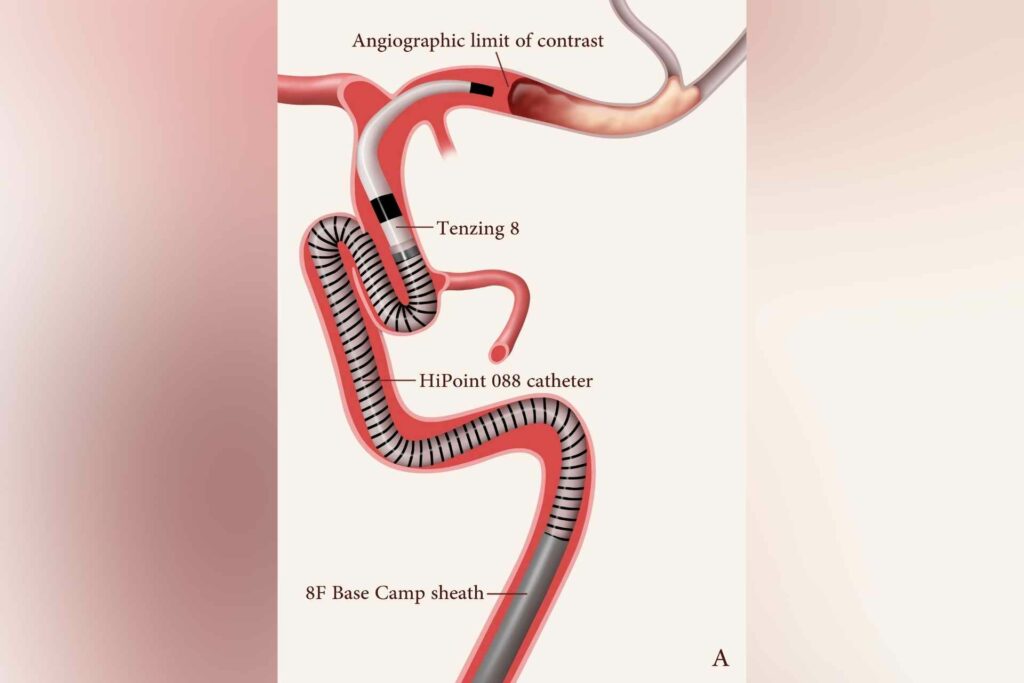
The SUMMIT MAX study is a randomized clinical trial built upon the recently published results of the SUMMIT NZ study, which demonstrated a very high rate of successful recanalization, or restoration of blood flow, of blocked blood vessels in acute stroke patients for the Route 92 Medical’s 088 Hipoint™ Catheter product.
Results of the study are also intended to provide clinical evidence to support an application for FDA clearance. Thanh Nguyen, M.D., from Boston Medical Center, Guilherme Dabus, M.D., from Miami Cardiac and Vascular Institute and Baptist Neuroscience Institute, and Ajit Puri, M.D., from Umass Memorial Medical Center are the principal investigators of the study.
“We are honored to partner with the SUMMIT MAX investigators to begin our pivotal clinical trial and look forward to continued enrollment as the study progresses,” Tony Chou, M.D., Route 92 Medical co-founder and CEO, said. “We are confident that our Monopoint System featuring the 088 Hipoint™ and Tenzing™ Catheters is a platform positioned to transform neurovascular aspiration thrombectomy for large vessel occlusions.”
Route 92 Medical’s HIpoint™ Catheter, Tenzing™ Catheters, and Base Camp™ Sheath System received U.S. FDA 510(k) clearance for neurovascular access in 2020 and have also received CE marking for first-line aspiration thrombectomy in the European Union.
Each year, strokes affect about 16 million people and kill an estimated 6 million people globally. In the United States, more than 800,000 patients suffer from acute ischemic stroke each year, with an annual healthcare cost of $104 billion. Stroke is a critically time-sensitive disease, and without appropriate diagnosis and treatment, a majority of patients suffers permanent disability or death. Despite recent advances in life-saving endovascular treatment, only approximately 10 percent of eligible stroke patients are treated endovascularly today.
About Route 92 Medical, Inc.
Based in San Mateo, CA, Route 92 Medical’s mission is to become the leader in neurovascular aspiration thrombectomy. The Route 92 Medical platform utilizes the Monopoint operating system including an 088 catheter delivered to the neurovasculature using the specialized Tenzing catheter. The system is designed to provide superior navigation to challenging anatomy and robust support, along with unparalleled simplicity and speed.
For more information on the WVU Rockefeller Neuroscience Institute, visit WVUMedicine.org/RNI.